Puromycin, an analog of the 3′ end of aminoacyl-tRNA, causes premature termination of translation by being linked non-specifically to growing polypeptide chains. Here we report the interesting phenomenon that puromycin acting as a non-inhibitor at very low concentration (e.g. 0.04µM) can bond only to full-length protein at the C-terminus. This was proved by using a carboxypeptidase digestion assay of the products obtained by Escherichia coli cell-free translation of human tau 4 repeat (tau4R) mRNA in the presence of low concentrations of puromycin or its derivatives. The tau4R mRNA was modified to code for three C-terminal methionines, which were radioactively labeled, followed by a stop codon. The translation products could not be digested by carboxy-peptidase if puromycin or a derivative was present at the C-terminus of full-length tau4R. Puromycin and its derivatives at 0.04–1.0 µM bonded to 7–21% of full-length tau4R, depending on the ability to act as acceptor substrates. Furthermore, the bonding efficiency of a puromycin derivative to tau4R was decreased by addition of release factors. These results suggest that puromycin and its derivatives at concentrations lower than those able to compete effectively with aminoacyl-tRNA can bond specifically to full-length protein at a stop codon. This specific bonding of puromycin to full-length protein should be useful for in vitro selection of proteins and for in vitro and in vivo C-terminal end protein labeling.
Figure 7. A possible model of specific bonding of puromycin to full-length protein. (A) Non-specific bonding to nascent protein in competition with aminoacyl-tRNA at higher concentrations of puromycin. (B) Specific bonding to full-length protein at the stop codon, not in competition with aminoacyl-tRNA, at lower concentrations of puromycin. Puro stands for puromycin.
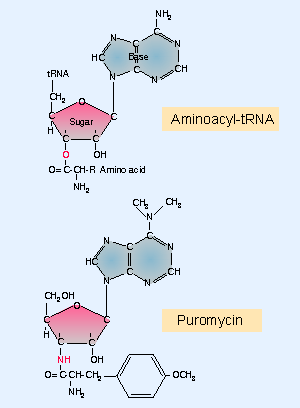 |
Figure 6.22 Puromycin mimics aminoacyl-tRNA because it resembles an aromatic amino acid linked to a sugar-base moiety. |
The nature of the transfer reaction is revealed by the ability of the antibiotic puromycin to inhibit protein synthesis. Puromycin resembles an amino acid attached to the terminal adenosine of tRNA. Figure 6.22 shows that puromycin has an N instead of the O that joins an amino acid to tRNA. The antibiotic is treated by the ribosome as though it were an incoming aminoacyl-tRNA. Then the polypeptide attached to peptidyl-tRNA is transferred to the NH2 group of the puromycin.
Because the puromycin moiety is not anchored to the A site of the ribosome, the polypeptidyl-puromycin adduct is released from the ribosome in the form of polypeptidyl-puromycin. This premature termination of protein synthesis is responsible for the lethal action of the antibiotic.
Peptidyl transferase is a function of the large (50S or 60S) ribosomal subunit. The transferase is part of a ribosomal site at which the ends of the peptidyl-tRNA and aminoacyl-tRNA are brought close together. Both rRNA and 50S subunit proteins are necessary for this activity. The catalytic activity is a property of the ribosomal RNA of the 50S subunit.
gene IV
'WET > Priciples' 카테고리의 다른 글
| 세포배양 (0) | 2015.08.02 |
|---|---|
| Recombinant Protein (0) | 2015.05.07 |
| MALDI_from googling (0) | 2015.04.23 |
| Formaldehyde, a cross-linker forming schiff base (0) | 2015.04.04 |
| Cyclohexamide(CHX) as a translation elongation inhibitor in Eukaryotes (0) | 2015.04.04 |



